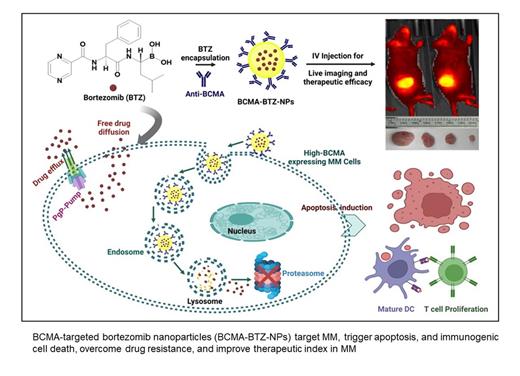
Bortezomib (BTZ) is a standard-of-care treatment for multiple myeloma (MM). In addition to its direct anti-MM effects, we recently reported that BTZ triggers immunogenic cell death associated with improved outcome. However, adverse effects limit its long-term clinical use. To improve its therapeutic index, we encapsulated bortezomib in PEG-PLGA nanoparticles linked with antibodies that target B-cell maturation antigen (BCMA-BTZ-NPs) to specifically target MM cells. BCMA-BTZ-NPs showed significantly increased cellular internalization into wild-type (WT) H929 cells after 6h (99.2±0.7%) than non-targeted NPs (57.7±0.8%) (p<0.0001), with a time-dependent increase in internalization. Importantly, cellular internalization of the BCMA-BTZ-NPs in BCMA-KO H929 cells (15.9±2.5%) was significantly depleted (p<0.0001). BCMA-BTZ-NPs showed target-specific cytotoxicity against MM cell lines and primary tumor cells from MM patients. Furthermore, BCMA-BTZ-NPs induced cell death more efficiently than non-targeted nanoparticles or free BTZ, triggering potent mitochondrial depolarization assessed by JC1 staining in MM.1S (79.06±3.7% vs. 57.4±1.9% for BCMA-BTZ-NPs vs non-targeted NPs, p<0.01); and H929 cells (47.23±1.9% vs. 31.26±0.7% for BCMA-BTZ-NPs vs. non-targeted NPs, p<0.001). This was associated with enhanced activation of caspases 8, 9 and 3, followed by apoptosis. We also found that BCMA-BTZ-NPs were still effective in BTZ-resistant MM cell lines (36.66±1.8% residual chymotryptic activity in RPMI-Dox40 [resistant] and 40.29±1.0% in MM.1S [sensitive] cells), whereas free BTZ showed no inhibitory effect (100.01±4.6% residual chymotryptic activity in RPMI-Dox40), likely due to BCMA-BTZ-NPs entering the cell through receptor-mediated uptake which averts acquired BTZ resistance based on the drug pump P-glycoprotein (PgP). Importantly, BCMA-BTZ-NPs also enhanced immunogenic cell death (ICD) and activated the autophagic pathway more than free BTZ, evidenced by increased calreticulin cell surface exposure (free BTZ: 45.13±3.7% vs BCMA-BTZ-NP: 71.5±3.4%,) and induction of T-cell proliferation in both MM.1S (free BTZ: 36.5±2.6% vs. BCMA-BTZ-NP: 65.2±2.3%, p<0.001) and AMO-1 cells (free BTZ: 37.03±1.3% vs. BCMA-BTZ-NP: 66.33±3.3%, p<0.001). Of note, increased levels of TNFα, Granzyme B, and IFNγ were also present in BCMA-BTZ-NPs treated MM cells with DC (Dendritic cells) and T cells co-culture supernatants.
Finally, we evaluated the in vivo target selectivity and therapeutic efficacy of BCMA-BTZ-NPs against human MM cells in a murine xenograft model. BCMA-BTZ-NPs selectively accumulated in the tumor more efficiently than non-targeted BTZ-NPs and were associated with more efficient tumor reduction and host survival. In summary, our study indicates that BCMA-BTZ nanotherapy accumulates at the tumor site, enhances therapeutic efficacy, triggers immunogenic cell death, overcomes drug resistance, and reduces off-target toxicity. These results provide the framework for clinical evaluation of BCMA-BTZ-NPs to increase the therapeutic index of BTZ and improve patient outcome in MM.
Disclosures
Anderson:Pfizer, Janssen, Astrazeneca, Daewoong, Amgen, Starton, OncoPep, Precision Biosciences, Window Therapeutics, Mana Therapeutics: Membership on an entity's Board of Directors or advisory committees; Window, Starton: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; NextRNA: Current equity holder in private company; C4 Therapeutics, Raqia, NextRNA,Dynamic Cell Therapy: Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Dynamic Cell Therapies: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Oncopep: Current equity holder in private company, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal